Global Compliance
Product quality is a fundamental part of our operational philosophy. OncoGenerix’s process and facilities have been implemented to meet the requirements of the CFDA, US FDA and EMA regulatory requirements for clinical and commercial supplies.
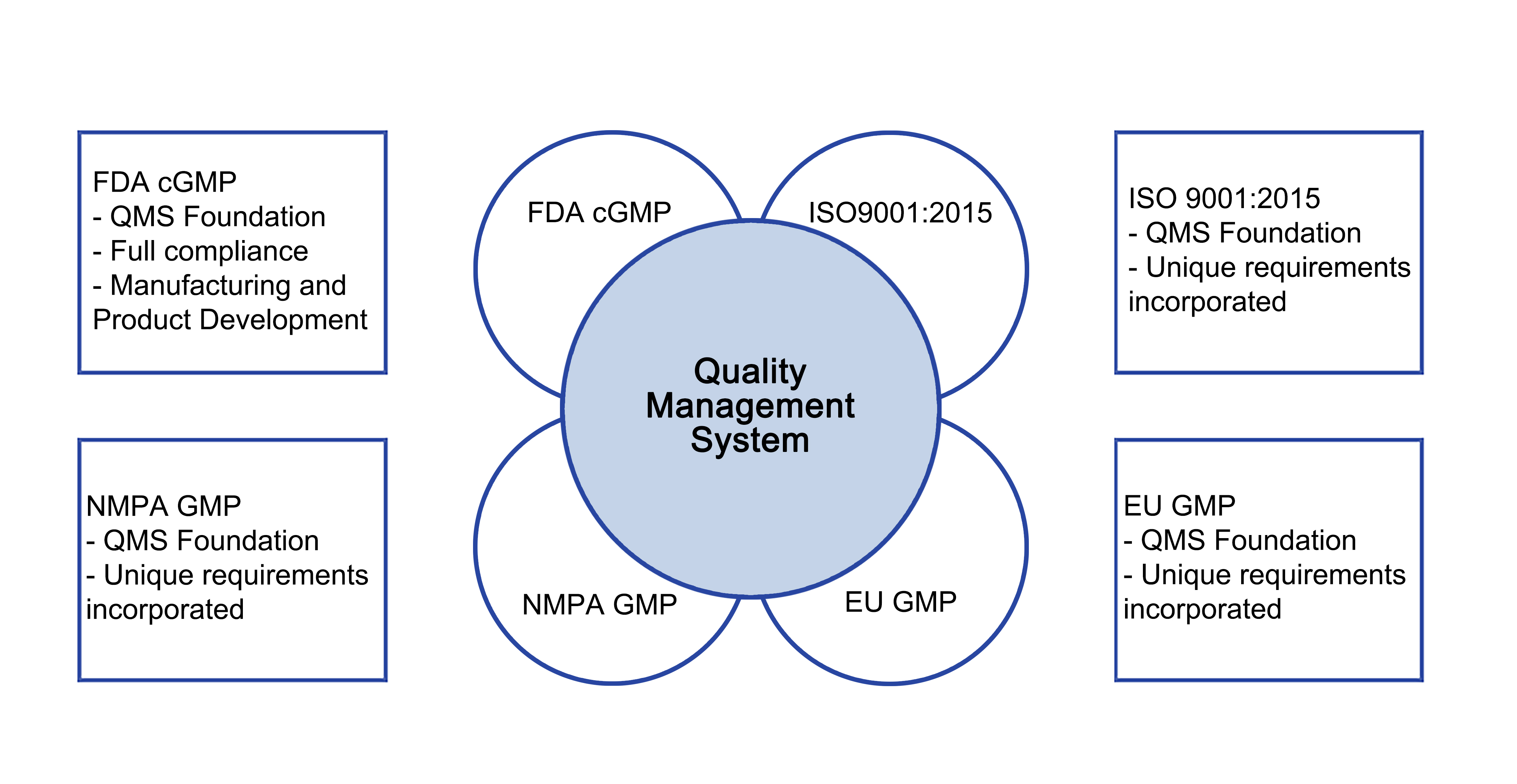
Quality Management System
OncoGenerix’s Quality Management System (QMS) has been designed and implemented to meet the quality requirements of the CFDA, US FDA and EMA and is based on the following key standards and guidelines:
- CFDA, Good Manufacturing Practice (2010 Revision).
- 21 CFR Parts 210, “Current Good Manufacturing Practices in Manufacturing, Processing, Packing, or Holding of Drugs.”
- 21 CFR Parts 211, “Current Good Manufacturing Practices for Finished Pharmaceuticals.”
- 21 CFR Part 11, “Electronic Records, Electronic Signatures.”
- ISO 9001: 2015, “Quality Management Systems Requirements.”
- EudralLex Volume 4, “EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use.”
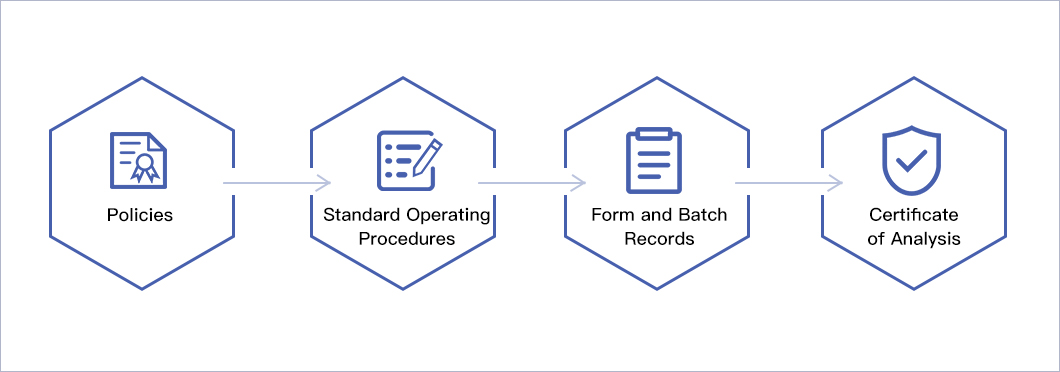
The quality management system is based on the following documentation hierarchy:
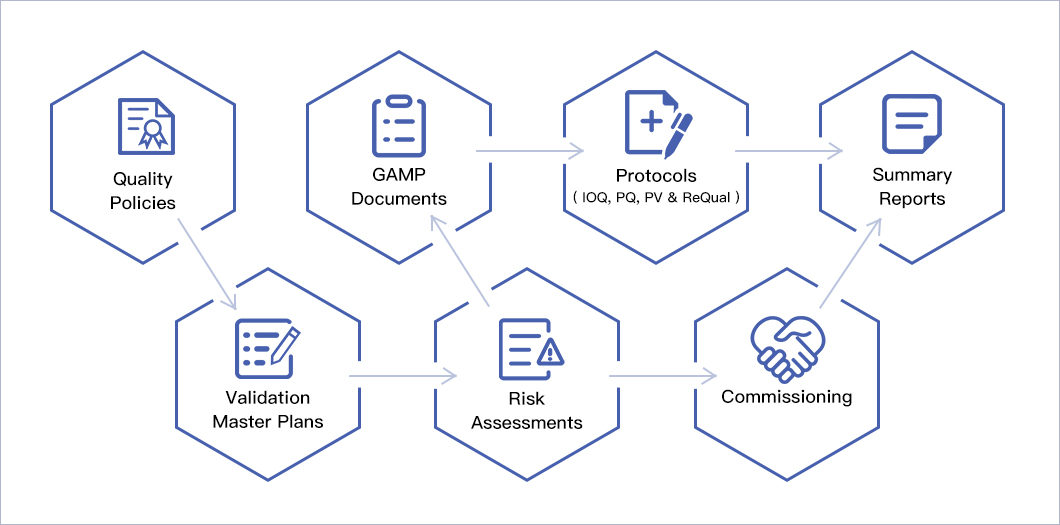
Facility / Process Implementation Strategy
The facilities and processes at OncoGenerix have been implemented using a QbD risk-based approach (FDA Process Validation 2011, ICH Q8, ICH Q9, ICH Q10, GAMP, ISPE Commissioning & Qualification Baseline, 21 CFR 211 and EU GMP regulations) within a Defined Life Cycle (ASTM E2500) for implementation, qualification and validation of process and facilities. This approach uses the highest quality and compliance standards resulting in products that fully meet the clinical needs of the patient. The facility and process implementation, qualification, validation, and revalidation are based on the following hierarchy: